Total Dissolved Solids In Water

Water is a good solvent and picks up impurities easily. Pure water -- tasteless, colorless, and odorless -- is often called the universal solvent. Dissolved solids" refer to any minerals, salts, metals, cations or anions dissolved in water. Total dissolved solids (TDS) comprise inorganic salts (principally calcium, magnesium, potassium, sodium, bicarbonates, chlorides, and sulfates) and some small amounts of organic matter that are dissolved in water.
TDS in drinking-water originate from natural sources, sewage, urban run-off, industrial wastewater, and chemicals used in the water treatment process, and the nature of the piping or hardware used to convey the water, i.e., the plumbing. In the United States, elevated TDS has been due to natural environmental features such as mineral springs, carbonate deposits, salt deposits, and sea water intrusion, but other sources may include: salts used for road de-icing, anti-skid materials, drinking water treatment chemicals, stormwater, and agricultural runoff, and point/non-point wastewater discharges.
In general, the total dissolved solids concentration is the sum of the cations (positively charged) and anions (negatively charged) ions in the water. Therefore, the total dissolved solids test provides a qualitative measure of the amount of dissolved ions but does not tell us the nature or ion relationships. In addition, the test does not provide us insight into the specific water quality issues, such as Elevated Hardness, Salty Taste, or Corrosiveness. Therefore, the total dissolved solids test is used as an indicator test to determine the general quality of the water. The sources of total dissolved solids can include all of the dissolved cations and anions, but the following table can be used as a generalization of the relationship of TDS to water quality problems.
Cations combined with Carbonates
CaCO3, MgCO3 etcAssociated with hardness, scale formation, bitter tasteCations combined with Chloride
NaCl, KClSalty or brackish taste, increase corrosivity
An elevated total dissolved solids (TDS) concentration is not a health hazard. The TDS concentration is a secondary drinking water standard and, therefore, is regulated because it is more of an aesthetic rather than a health hazard. An elevated TDS indicates the following:
1)The concentration of the dissolved ions may cause the water to be corrosive, salty or brackish taste, result in scale formation, and interfere and decrease efficiency of hot water heaters; and
2)Many contain elevated levels of ions that are above the Primary or Secondary Drinking Water Standards, such as an elevated level of nitrate, arsenic, aluminum, copper, lead, etc.
Total Dissolved Solids (TDS): In a laboratory setting, the total dissolved solids is determined by filtering a measured volume of sample through a standard glass fiber filter. The filtrate (i.e., filtered liquid) is then added to a preweighed ceramic dish that is placed in a drying oven at a temperature of 103 C. After the sample dries, the temperature is increased to 180 C to remove an occluded water, i.e., water molecules trapped in mineral matrix. Total dissolved solids means the total dissolved (filterable) solids as determined by use of the method specified in Title 40 of the Code of Federal Regulations (40 CFR) Part 136.
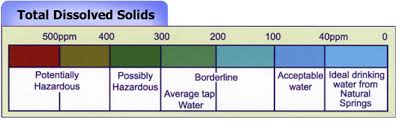
High total dissolved solids may affect the aesthetic quality of the water, interfere with washing clothes and corroding plumbing fixtures. For aesthetic reasons, a limit of 500 mg/l (milligrams per liter) has been established as part of the Secondary Drinking Water Standards.
An approximation of the Total Dissolved Solids:
A. The total dissolved solids concentration can be related to the conductivity of the water, but the relationship is not a constant. The relationship between total dissolved solids and conductivity is a function of the type and nature of the dissolved cations and anions in the water and possible the nature of any suspended materials. For example, a NaCl solution and KCl solution with a conductivity of 10000 umhos/cm will not have the sample concentration of NaCl or KCl and they will have different total dissolved solids concentration. Conductivity is measured through the use of a meter and is usually about 100 times the total cations or anions expressed as equivalents and the total dissolved solids (TDS) in ppm usually ranges from 0.5 to 1.0 times the electrical conductivity.
In addition, water supplies can contain dissolved organic chemical contaminants which are usually pollutants that enter water as a result of man's activities, such as insecticides, pesticides and herbicides. These are usually chronically, rather than acutely, toxic to man and other species in extremely small amounts. Trihalomethanes are dissolved organic contaminants, such as chloroform, which are formed in extremely small amounts by the reaction of chlorine used to disinfect water, with humic and fulvic acids from soil erosion. Other organics can enter both surface and groundwater through waste dumping, such as trichlorethylene, tetrachlorethylene (TCEs), polychlorinated biphenyls (PCBs), dioxin, etc. Many of the organic contaminants are probably carcinogenic (cancer-producing). The organics do not necessarily exist in water in the form of dissolved ions.
The Secondary Drinking Water Regulations control contaminants in drinking water that primarily affect the aesthetic qualities of water. Several of these -- chloride, sulfate, copper, iron, manganese, zinc, and total dissolved solids -- are ionized contaminants.
Color and odor are contaminants which cause objectionable sensory responses to the water.
pH is a measure of the acid or alkaline strength of a water supply and corrosivity refers to the ability of a water supply to disintegrate pipes and containers.
Why should you measure TDS levels in your water?
When TDS levels exceed 1,000 ppm (parts per million) it is deemed unfit for human consumption. A high level of TDS is an indicator of potential concerns and should be investigated before drinking. Even the best water purification systems on the market require monitoring for TDS to ensure the filters and/or membranes are effectively removing unwanted particles from your water.
We are all affected by toxic chemicals in the air and food that we consume. Water is the only way to flush out these toxins so it is important to make sure your water source is providing pure water. This is especially important for children because they are much more sensitive to contaminants because their defense systems have not fully developed. The purer the water is the greater its ability to purify and cleanse the body, so drink up!
Total Dissolved Solids can be measured in the field using an electronic pen. Many of these devices actually measure the conductivity of the water, i.e., the ability of the water to carry a charge, and not the actual total dissolved solids. These devices then calculate the total dissolved solids assuming that the primary dissolved minerals are either a combination of NaCl or KCl. Therefore, the measurement of total dissolved solids by these devices are not an accurate measure, but an approximation. If you are thinking of using these devices for a project, I would recommend purchasing a conductivity pen which measures the conductivity of the water.